Redefining What’s Possible in Microsurgery: How MMI Brought a Robotic Surgical System to Market in Record Time
CUSTOMER STORY
LIFE SCIENCES | 5 MINUTE READ
MMI utilizes the NI platform to rapidly develop the Symani® robotic surgical system, transforming microsurgery worldwide with precision and speed.
In the intricate world of microsurgery, where precision is paramount and the slightest misstep can have serious consequences, Medical Microinstruments (MMI) stands at the forefront of innovation. Founded in 2015 near Pisa, Italy, MMI embarked on a mission to enhance surgical performance through the use of advanced robotic systems.
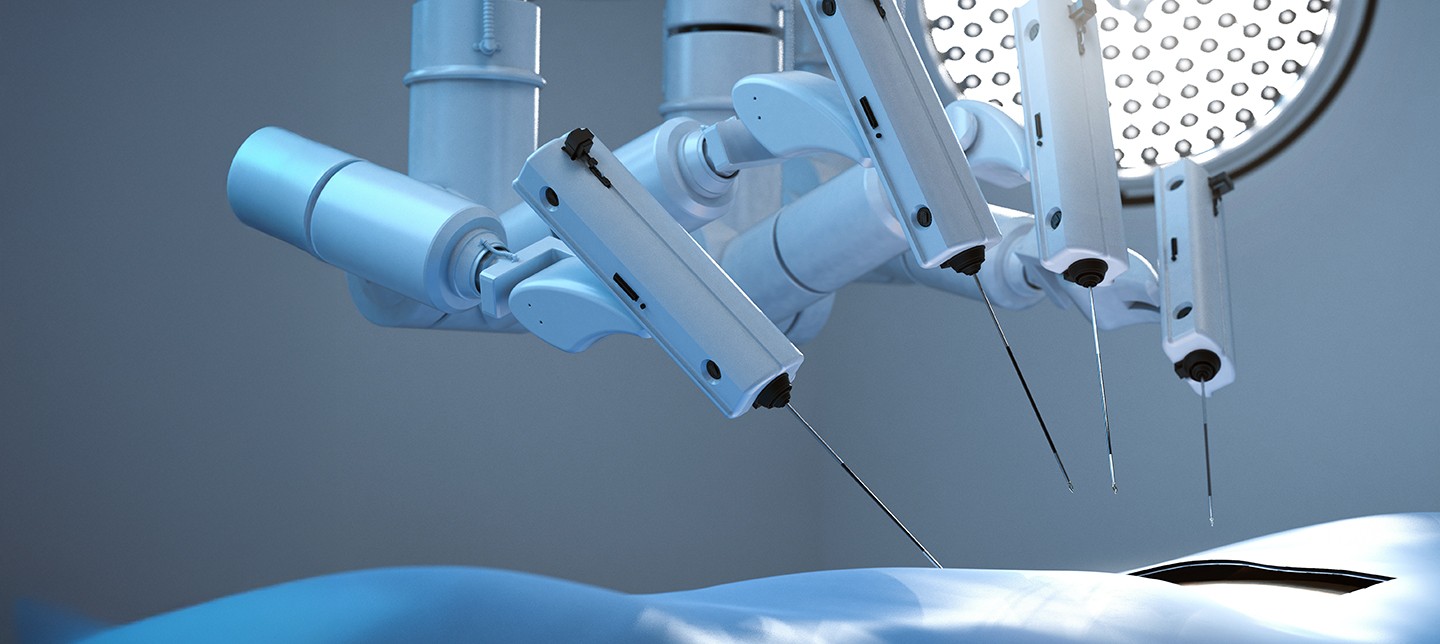
Their flagship product, the Symani® Surgical System, combines the world’s smallest-wristed microinstruments with tremor-reducing and motion-scaling technologies, empowering surgeons to perform delicate procedures with unprecedented accuracy.
Reimagining Microsurgery
Human dexterity and fine motor control often begin to decline in the mid-forties. This physiological reality presents a unique challenge in the operating room: just as surgeons reach the peak of their expertise, they may find their precision subtly diminished.
Microsurgery poses an exceptionally demanding challenge, particularly in procedures like anastomosis, where surgeons must join tubular structures, such as blood vessels, that can be as thin as 0.3 mm, narrower than a business card. These operations demand extraordinary steadiness and control, pushing the limits of even the most skilled hands.
MMI envisioned a way for surgeons to restore the quality of life for more patients with complex conditions. However, achieving this goal required more than breakthrough surgical innovation—it demanded a system that could perform flawlessly in high-stakes medical environments, adapt quickly to clinical feedback, and comply with stringent global regulatory standards.
A Flexible, Software-Connected Platform Built for Iteration
To move quickly from concept to commercial readiness, MMI needed a development platform that could deliver real-time performance without sacrificing precision, safety, or compliance.
They selected NI CompactRIO hardware, along with the NI LabVIEW Real-Time Module and the NI LabVIEW FPGA Module, to build the foundation of Symani’s embedded control system. These tools enabled MMI to develop a responsive and scalable architecture that could evolve alongside the product and meet rigorous regulatory requirements. The intuitive graphical interface in LabVIEW significantly reduced the learning curve, allowing the team to move quickly. The LabVIEW FPGA Module simplified development that would have otherwise required hundreds of lines of HDL code, and the LabVIEW Real-Time Module helped the team accelerate development while ensuring consistent performance.
By choosing the NI platform, MMI achieved faster integration, simplified scaling, and fewer points of failure—all of which helped MMI develop a sophisticated medical device in a short timeframe with a small engineering team.
From Start-Up to Global Surgical Impact in Just Four Years
Thanks to the NI integrated platform, MMI achieved remarkable success in a short timeframe, particularly for a company operating in the highly regulated medical field. With FDA authorization in the United States and CE Mark certification in Europe, Symani is redefining what’s possible in microsurgery and bringing new hope to patients worldwide.
To date, the Symani Surgical System has been utilized in more than 1,500 successful human procedures and is globally recognized as a transformative innovation in microsurgery. It even earned a coveted spot on the TIME Magazine Best Inventions of 2024 list.
By combining NI modular hardware and open software tools with their own deep expertise in surgery and engineering, MMI turned a bold vision into life-changing medical technology, faster than anyone thought possible.
Looking Ahead
MMI is expanding the scope of Symani to support additional procedures and certifications as demand for precision surgical robotics continues to grow. As they look to the future, they remain confident in the NI platform and are excited to adopt the next generation of CompactRIO hardware to further enhance system performance and control.